How to employ phenotyping of photosynthesis-related parameters to measure stress-tolerance in crops.
Tuesday 29th June 2021
12.30pm-17.00pm CEST
More about our speakers

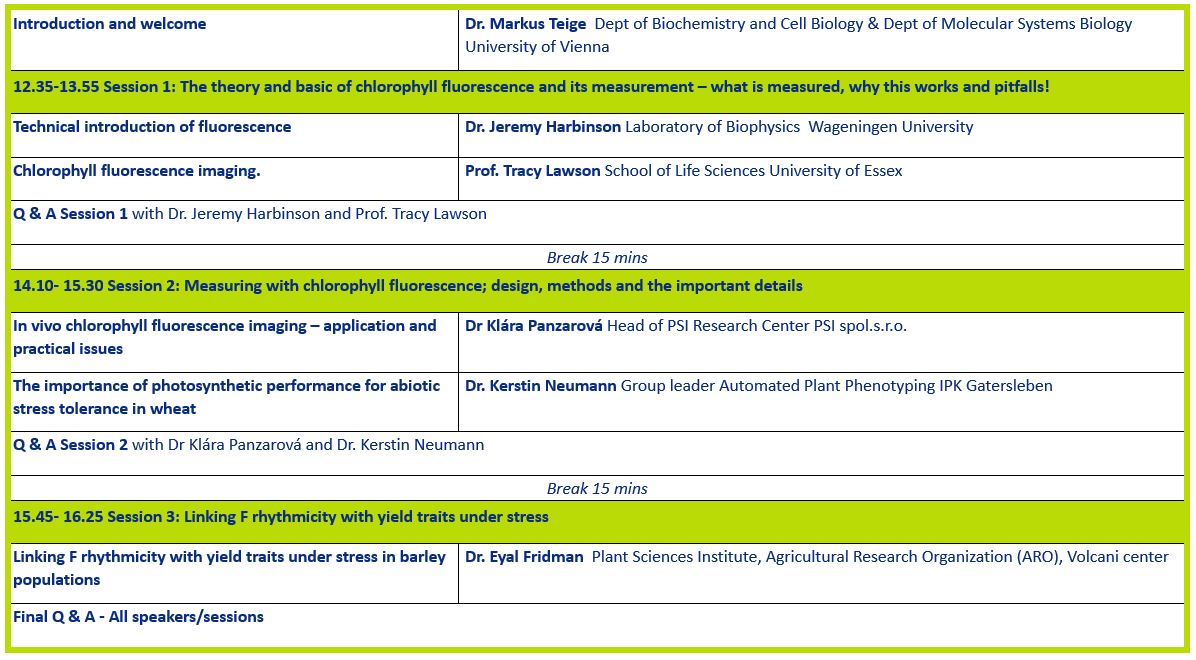
Dr Jeremy Harbinson
Chlorophyll fluorescence; what is it and how does it work as a probe of photosynthesis
Abstract:The use of chlorophyll fluorescence to monitor photosynthesis, and in particular processes within photosystem II, hinges upon the role played by the excited state of chlorophyll in photosynthesis. This excited state is essential to photosynthesis and the nearly all the energy that drives life on Earth has at some point been carried by an excited chlorophyll molecule. These excited states are also the source of chlorophyll fluorescence and understanding their nature and fate in photosynthesis underpins the use of chlorophyll fluorescence as a way to probe, in detail, photosynthesis. In my talk I will give a summary of the basic physics (can’t avoid that!) and biology of fluorescence, and the excited state of chlorophyll, to show why fluorescence is used as it is. I will also say something about how fluorescence is measured and why these methods are needed. Finally I will say something about some basic applications of fluorescence and some of the things to be aware when using fluorescence-based parameters.
Further reading:
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. Journal of Experimental Botany 64: 3983–3998
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Rev Plant Biol 59: 89–113
Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30: 1107–1125
Harbinson J (2018) Chlorophyll fluorescence as a tool for describing the operation and regulation of photosynthesis in vivo. In R Croce, RV Grondelle, HV Amerongen, IV Stokkum, eds, Light Harvesting in Photosynthesis, CRC Press,
van Bezouw RFHM, Keurentjes JJB, Harbinson J, Aarts MGM (2019) Converging phenomics and genomics to study natural variation in plant photosynthetic efficiency. The Plant Journal 97: 112–133

Prof Tracy Lawson
Chlorophyll fluorescence imaging.
Abstract: Chlorophyll fluorescence is a non-invasive measurement of photosystem II (PSII) activity and is commonly used to explore various aspects of plant performance. It is becoming increasingly used as a high throughput screening tool. As the end product of electron transport feeds directly into carbon fixation, and this forms the basis for nearly all plant metabolism, perturbation of metabolic processes that are not directly involved in photosynthesis can also alter fluorescence signatures (Baker 2008), extending the value of chlorophyll fluorescence as a phenotyping tool. The development of chlorophyll fluorescence imaging not only enables rapid high throughput screening, but also enables both spatial and temporal variation to be assessed. Furthermore combining chlorophyll fluorescence with other physiological tools (e.g. infra-red gas exchange analysis, thermography) allows us to obtain a greater mechanistic understanding of what drives differences in photosynthetic efficiency. In this workshop we will cover the basic principles as well as combined approaches for phenotyping.
Bio: Tracy is a plant physiologist with considerable experience in photosynthetic physiology and phenotyping with a special focus on stomatal behaviour. Some of her research has included the development of novel phenotyping tools and approaches to screen plant performance, including the development of the first imaging system to measure dynamic plant water use efficiency (McAusland et al., 2013). Lawson is author/co-author on >125 publications and was cited on the world highest cited list in 2019. She is also Director of Plant Phenotyping and Director of the Essex Plant Innovation Centre (EPIC) which brings scientists together with industry to address some local and global challenges facing the agriculture and horticultural sector.
E.H. Murchie, T. Lawson. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. Journal of Experimental Botany, Volume 64, Issue 13, October 2013, Pages 3983–3998, https://doi.org/10.1093/jxb/ert208
Baker, N.R., 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu. Rev. Plant Biol., 59, pp.89-113.
Maxwell, K. and Johnson, G.N., 2000. Chlorophyll fluorescence—a practical guide. Journal of experimental botany, 51(345), pp.659-668.

Dr Klára Panzarová
In vivo chlorophyll fluorescence imaging – application and practical issues
Abstract: The aim of this presentation is to provide practical information on the hardware, methodology, and the hands-on application of chlorophyll (Chl) a fluorescence imaging technique in plant image-based phenotyping field. Brief technical overview of state of art solutions in Chl fluorescence imaging will be provided, however the main focus of the presentation is to provide overview of possible applications for ChlF imaging technique in non-invasive scoring of plant performance during various conditions and in different plant species. In addition, practical aspects on how to properly design experimental protocol will be discussed.
Bio: Dr. Klára Panzarová is a plant biologist working as chief scientist for technical support in PSI company (Photon Systems Instruments). She is the head PSI Research Center since beginning of 2018. She has a long-standing expertise in development of experimental protocols and in using image-based techniques for non-invasive analysis of morpho-physiological traits in different crops in different conditions.
Suggested Back ground reading:
E.H. Murchie, T. Lawson. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. Journal of Experimental Botany, Volume 64, Issue 13, October 2013, Pages 3983–3998, https://doi.org/10.1093/jxb/ert208
Kalaji, H.M., Schansker, G., Brestic, M. et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth Res 132, 13–66 (2017). https://doi.org/10.1007/s11120-016-0318-y

Dr Kerstin Neumann
The importance of photosynthetic performance for abiotic stress tolerance in wheat
Abstract: The big challenge in agriculture is to ensure future yield and thereby food security for a growing human population under deteriorating climatic conditions and without the possibility to increase arable land. The optimization of photosynthesis may represent a valuable approach to improve productivity, in particular under abiotic stress conditions. Studies have been conducted with winter and spring wheat collections to evaluate photosynthesis-related parameters in combination with high-throughput non-invasive phenotyping. In particular under stress, there is a high phenotypic variation and thereby room for crop improvement. In combination with genomic information, genome wide association studies have been conducted that lead to the identification of quantitative trait loci (QTL) and potential candidate genes, which link photosynthesis to other important phenotypic traits. Results also point to a relationship of photosynthetic performance in HTP and crop performance in the field, underlining the breeding relevance of these traits.
Bio:Dr. Kerstin Neumann is a biologist and is leading the group “Automated Plant Phenotyping” at IPK since beginning of 2021. She has a long-standing expertise in the development of phenotyping setups for non-invasive high throughput phenotyping (HTP) systems and their application to evaluate diverse cereal collections for abiotic stress tolerance (drought and heat) by working as a Postdoc at IPK since 2010 with one of the HTP systems. The generated phenotypic data are utilized to elucidate trait genetic architecture and resolve their temporal dynamics and provide insights into trait relationships and trade-offs. The main goal is to improve the performance of crops under abiotic stress which is increasing due to the ongoing climate change.
Suggested Back ground reading:
Grieco M, Roustan V, Dermendjiev G, Rantala S, Jain A, Leonardelli M, Neumann K, Berger V, Engelmeier D, Bachmann G, Ebersberger I, Aro E-M, Weckwerth W, Teige M (2020) Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant Cell Environ. 43 1484-1500. dx.doi.org/10.1111/pce.13756
Dhanagond S, Liu G, Zhao Y, Chen D, Grieco M, Reif J, Kilian B, Graner A, Neumann K (2019) Non-invasive phenotyping reveals genomic regions involved in pre-anthesis drought tolerance and recovery in spring barley. Front. Plant Sci. 10 (2019) 1307. dx.doi.org/10.3389/fpls.2019.01307

Dr Eyal Fridman
Linking F rhythmicity with yield traits under stress in barley populations
Abstract: The plasticity and stability of plant phenotype towards changes in the environments plays a key role in their adaptation. Rhythmicity based on Chl leaf fluorescence (F) is a non-invasive and relatively high-throughput measurement that provides estimates of period and amplitude of the plant circadian clock output. It could possibly link early physiological processes, i.e. photosynthesis, with final agricultural phenotypes due to relevant source-sink relationship between vegetative and reproductive tissues. We attempt to gain better understanding of these links by correlating F and field measured phenotypes of mapping and diversity panels of barley, and by identifying pleiotropic quantitative trait loci (QTL) that affect both type of traits. Since F phenotype are chloroplast-based processes we also examine possible effects of cytoplasm DNA diversity on rhythmicity. In this talk I will present genome scans performed in barley populations to identify pleiotropic loci regulating the clock and field phenotypes under ambient and high temperatures. I will also discuss possible predictive models between F rhythmicity and yield components phenotypes.
Bio: I completed my PhD degree at the Hebrew University under the supervision of Prof Dani Zamir in 2002, during which I was able to clone the first QTL in any organism and explore evolution and physiology related to the underlying invertase gene. I then spent three years at the group of Prof Eran Pichersky at the University of Michigan, exploring specialized metabolism and unravelling hitherto unknown genes and enzymatic reactions in several pathways. Since 2005 I lead a research group, initially at the Faculty of Agriculture, the Hebrew University, and since 2014 at the Plant Sciences Institute at the Volcani Center ARO. My group have studied sources of phenotypic variation, the genetic basis for these phenotypes and the role GxE plays in shaping plasticity, stability and eventually adaption. On the genetic front, we develop mapping populations, and allele mining approaches to mine them for causal variation. We also explore the utilization of CRISPR/CAS9 for fine mapping of diversity underlying complex traits in crop plants.
Suggested Back ground reading:
Prusty MR, Bdolach E, Yamamoto E, Tiwari LD, Silberman R, Doron‐Faigenbaum A, Neyhart JL, Bonfil D, Kashkush K, Pillen K, et al (2021) Genetic loci mediating circadian clock output plasticity and crop productivity under barley domestication. New Phytol. doi: 10.1111/nph.17284
Bdolach E, Prusty MR, Faigenboim-Doron A, Filichkin T, Helgerson L, Schmid KJ, Greiner S, Fridman E (2019) Thermal plasticity of the circadian clock is under nuclear and cytoplasmic control in wild barley. Plant Cell Environ. doi: 10.1111/pce.13606
Hübner S, Bdolach E, Ein-Gedy S, Schmid KJ, Korol a., Fridman E (2013) Phenotypic landscapes: Phenological patterns in wild and cultivated barley. J Evol Biol 26: 163–174
A collaborative training workshop organised by:
ADAPT is funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No GA 2020 862-858